‘Audit’ – the mere mention of the word can strike fear into the heart of even the most experienced and organised of laboratory managers. You can spend hours – sometimes weeks – preparing for an external audit. You can spend time collating information, formatting documents, and ensuring your teams is ready to handle any questions that may be directed towards them. Thank goodness they only take place every couple of years! But what if you look at audits differently – as a means of improving lab and sample quality?
Sample tracking software and laboratory information management system (LIMS) can help you instil quality and traceability into your daily processes. The right software systems implemented with the relevant workflows can help ease some of your administrative burdens around data management for audit compliance.
What if you were to go one step further and carry out your own internal audits?
The thought of carrying out your own internal audits and quality control checks may bring you out in a cold sweat! You don’t have time to regularly spend hours getting your information together to check its quality. This is where sample tracking software can help – quickly pulling together your information and displaying in easily digestible formats. Within our sample tracking software, Achiever Medical, our oversight officer dashboard displays real-time information about consent and ethics to help you comply with legislation such as the Human Tissue Act (HTA). Using this information, for example, you can easily check if your consent withdrawal procedures are being carried out appropriately.
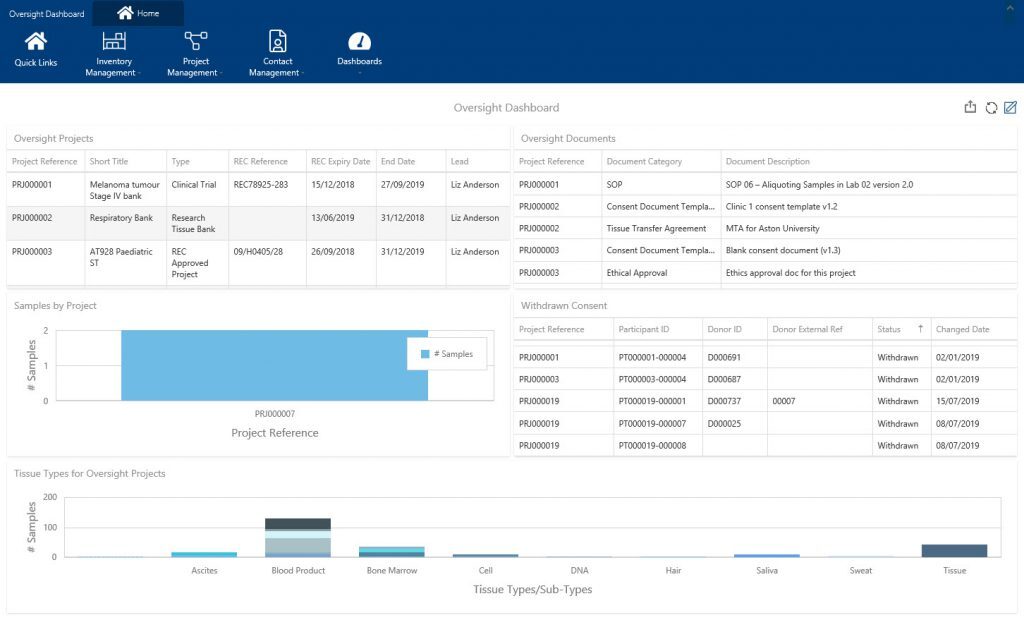
Whatever the frequency, if you actively conduct your own internal quality checks on your biospecimens and storage locations this can help eliminate the traditional ‘audit rush/panic’.
Regular internal audits and quality checks allow you to confirm that the procedures and processes you have implemented are working effectively and allow for earlier identification of improvements and any necessary corrective actions.
By carrying out your own audits you can help ease the tension that you usually experience around external audits. You and your team will be more confident demonstrating your quality processes and procedures. And importantly, be able to evidence their use.
Plus, your team will be well prepared and practised in the art of audits and understand the significance of quality.
What should you audit to help improve your lab and sample quality?
When deciding which areas to audit, you need to know the critical information you require to monitor and manage quality. You also need to be realistic. Some ideas of things to include in an internal audit are:
- Biological samples collected over a defined period to check their storage locations and current status.
- Biological samples that you have collected over a defined period to check their use is within agreed consent restrictions.
- Donors that have withdrawn consent to confirm all biological samples have been destroyed and no longer in your biorepository. You want to make sure you handle these in accordance with your Standard Operating Procedures (SOPs).
- Recording any compliance issues or required corrective action.
Tracking outcomes and progress
You can use integrated, interactive dashboards and reports to monitor audit progress and outcome. Dashboards can include audits completed, results (pass/fail), outcome comparisons (versus last year or last quarter), trends and averages. You can evidence this information in any external compliance audits such as those conducted by the Human Tissue Authority (HTA). In addition, you can make your data available for your management reporting. This can help you monitor and measure biospecimen viability, track Return on Investment (ROI) and increase the value of your biological samples.
A final thought about a proactive approach to auditing
Using sample tracking software to help you carry out your own internal audits means you can do these as and when you wish. Plus, integrated, intuitive dashboards continually organise your data, so the auditing process becomes simply a reporting exercise. As a result, your information is more readily available. This allows you to check as often as you like. And you are now taking a more proactive approach to improving lab and sample quality.

Comments are closed.