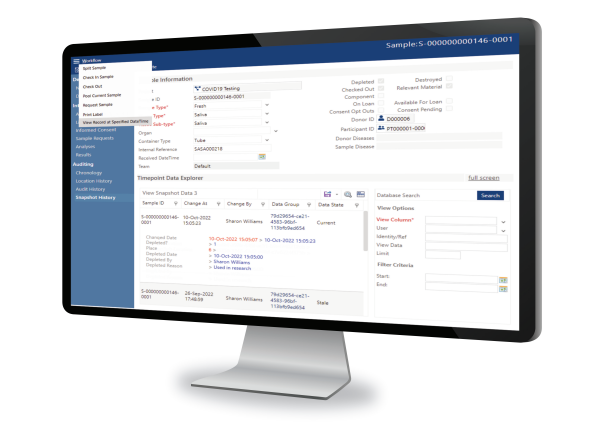
Using Achiever Medical LIMS to handle and process laboratory and patient information
Together with people, processes, systems and oversight, data management forms an integral part of regulatory and quality standards compliance. Critical decisions and outcomes rely on data. Consequently, making sure that all lab data is complete and accurate is an essential element of the auditing process.
However, with labs recording and processing increasing volumes of data, ensuring this data is being managed accurately and securely can be difficult. In addition to time-consuming. Plus, equally importantly, you must handle the data in line with patients’ wishes.
This requires all laboratory personnel involved with data management to be familiar with and understand the importance of data recording. In addition, those responsible for maintaining your organisation’s infrastructure and systems need to protect data from unauthorised access. Plus, lab managers, designated individuals, and oversight officers will need to be able to analyse data and identify any potential non-compliance issues quickly.
Achiever LIMS’ compliance features
Achiever LIMS’s offers a suite of tools to support all those involved in protecting, handling, and analysing your lab data.