Patient-centric approaches require patient clinical data to be held alongside sample data
You may already be aware of the benefits that Laboratory Information Management systems (LIMS) and sample management software can bring. When you are clear on what you need – and when implemented well – these systems can help you streamline your processes, improve sample quality and meet compliance. Most sample management and tracking systems are sample-centric – it’s all about the sample. And why wouldn’t they be? Isn’t that what they are designed for? True, but new approaches are emerging in investigative and clinical research. These new methods, such as precision and personalised medicine, focus on the individual patient. To become more patient-centric, you need to be able to store patient clinical data in Biobanking software, LIMS and sample management systems alongside your samples.
Our Achiever Medical customers have been securely storing patient clinical data alongside enhanced sample metadata for more than 10 years. We believe that the more you understand about your donors – their lifestyle choices, history and previous diagnoses – the more you can target your research. Plus, the better informed you are to spot trends or risks. LIMS and sample management systems that enable you to hold patient profiling data alongside your sample information can give your lab or Biobank invaluable insight.
Holding donor profile information while protecting confidentiality
Achiever Medical’s comprehensive donor management functionality allows you to record detailed patient clinical and lifestyle data – out of the box. Advanced security, through ‘at rest’, field-level data encryption, ensures only authorised users can view Patient Identifiable Information (PII) such as date of birth or surname. However, your non-authorised users continue to have access to profiling data, such as ethnicity, treatment and diagnosis. They can view and include this data when searching for samples to use as well as in any reports and analysis. Where users should not see sensitive data, the system displays the values as an asterisk. The screenshot below shows Achiever Medical’s donor search grid with the First Name and Surname columns as encrypted while other non-PII data is available for searching.
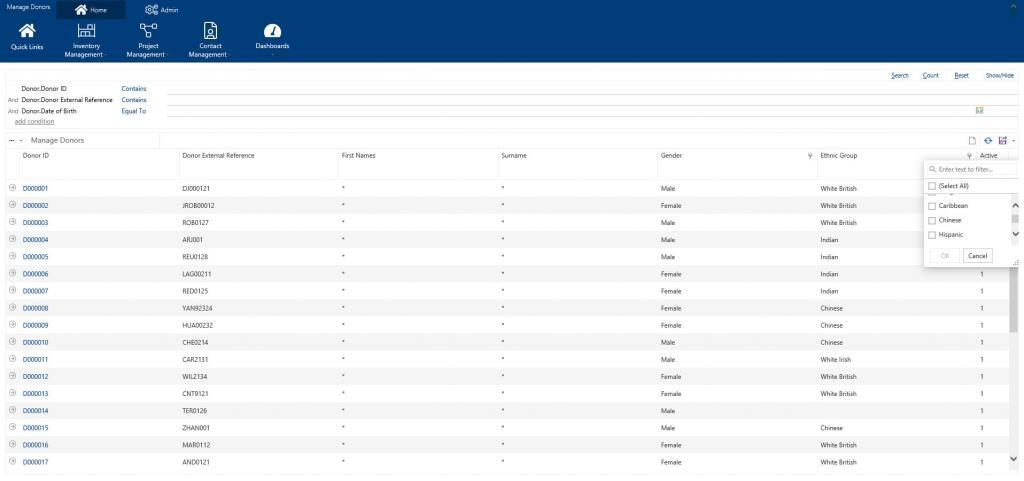
If you need to record more information, our flexible, easy-to-use configuration tools allow your super users to add fields. You can add these at anytime; when you need the information or it becomes available. You can search, query and import into your new fields as soon as you create them. And you can choose to encrypt them too.
Software to manage patient informed consent
Ensuring you have the appropriate donor informed consent for your research is critical. Not only from an ethical perspective but also a legal one. Achiever Medical’s enhanced informed consent management capabilities allow you to track active consent and any exclusions (opt-outs). Consent can be stored at patient, project, clinical trial and study level. Any opt-outs are highlighted on a donor’s samples, along with any missing consent that is required. Standard workflows automatically exclude donors that don’t have any active consent.
Plus, Achiever Medical’s withdraw consent process prompts users to make sure you get rid of all biological material associated with the donor. This also includes where the donor’s biological tissue is part of a Tissue Microarray (TMA). The software automatically audits the withdraw and destruction processes – helping you to meet compliance.
Software to track patient treatment and diagnosis data
Achiever Medical allows you to record diagnosis data against each donor. And it uses SNOMED and ICD codes to make sure information is standardised and consistent. You can also capture further information including patient treatment and medication history data. All this information is available for you to use in your searches and analysis. As a result, you can really target your sample selection for use in research.
Patient disease profiling and progression tracking system
Every disease is different – it may have different indicators, characteristics, risk-factors and stages. Unlike other LIMS and sample management systems, Achiever Medical enables you to track each disease and its associated data markers. Using Achiever Medical’s Disease Management module, you can set up and define as many diseases as you need. As you define each disease you create a disease profile which is made up of new fields you create to record the disease’s individual characteristics and stages.
In addition, you can record episode information to monitor and track disease progress.
Achiever Medical dynamically creates data-entry forms based on your disease profile. Both internal and external researchers can combine disease profiles with sample data when sourcing biological material for use.
Combining patient and biological sample information for enhanced investigative research
Holding patient clinical data in Biobanking software and sample management systems allows you to focus on your target donors as well as exclude samples or donors. Combining this data further with Achiever Medical’s informed consent management ensures that any sourced material complies with the appropriate consent.
If you are managing your clinical trials in Achiever Medical, you can use this data to analyse your trial participants. Plus, you can use it to identify potential participants for future trials.
Achiever Medical also gives you Customer Relationship Management (CRM) to further enhance your patient profiling and manage your suppliers and partners. Read more in our blog on the Advantages of CRM in Biobanking and Sample Management software.
Implementing or upgrading your LIMS or sample management system?
If you are upgrading your LIMS or Sample Management software, you may want to take the opportunity to review your patient and sample data processes. Take a look at our Next Generation Biobanks – Integrating Sample and Clinical Data blog. Why not take a look at Achiever Medical to see what it can give your team out of the box?
