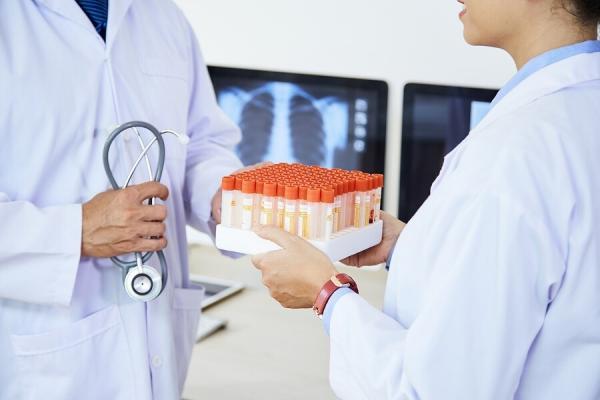
Two key challenges facing your lab or Biobank are quality and efficiency. You may introduce and follow a set of standard processes to address these. And, to be successful, everyone... read more →
When you use or implement any software system you hope it's going to improve how you do things and impact the quality and integrity of your data. When adopting new... read more →
For your Biobank to be effective you need researchers to be regularly requesting your tissue samples. This means you need to make them aware of your Biobank and what you... read more →
The thought of a lab audit might be enough to bring you out in a cold sweat. The dread of an audit for some is akin to a phobia. Desensitisation... read more →
Biobanks are the bridge between researchers and patients. Your job is to deliver high-quality biological specimens from willing participants to expert scientists. You are the conduit between those who want... read more →
Do you know what’s in your laboratory sample freezers and storage without opening them? If you dread the questions “Now, where did we put that sample?” or “What do you... read more →
A successful Biobank is one whose tissues are in demand and being used. If your samples are "flying off the shelves" then you're a real success! However, if demand is... read more →
Want to publish your samples to UKCRC Tissue Directory but don’t have the time? If you are one of the many UK Biobanks that wants to maximise visibility of their... read more →
When it comes to protecting your data and systems you will have security in place to stop external access and attacks. But recent events show that a security breach can... read more →
This week we saw another announcement in the news where personal data had been stolen. This time it was student data from a prestigious UK University. As discussed in our... read more →