Each sample’s journey is a complex one. A sample may be handled by many people as it moves from initial collection through various states and stages to final depletion. It may travel hundreds of miles. You may freeze and thaw it a couple of times. What’s more, you may split, slice, analyse and process it beyond recognition. So, when it comes to choosing a sample for testing, clinical trials or research are you confident it’s going to be viable? And can you trace everything that you’ve done to it. With Achiever Medical laboratory information management system (LIMS) you can track and manage the movement of every sample. As a result, you know exactly where each sample came from, where it’s been, who’s done what to it, where it is now and, importantly, how you can ethically use it.
Do you really know your sample’s origin?
Understanding a sample’s provenance can help you assess its viability. Knowing exactly why a sample was taken, where it was taken and how it’s being stored are just the first steps to understanding if you can use it. Then there’s the informed consent. This details a patient’s wishes in how they want you to use or, equally as important, not use their samples.
Achiever Medical LIMS enables you to track all this information and find it quickly and easily whenever you or researchers need it. Every time you record a sample in the LIMS you can enter its collection date, time, location and storage data. You can also link it against its corresponding patient record. What’s more, you can hold the patient’s informed consent details including any restrictions and expiry dates. As a result, you’re able to see all samples a patient has donated. Plus, you can check that you’re using your samples ethically as well as in compliance with any regulatory requirements. Above all, you’re making sure you’re complying with the patient’s wishes.
Every time you check a sample in or out of storage the LIMS automatically creates an audit record that you can easily view. So along with all the original sample and patient consent information you can also check if another person has already used the sample. And why.
Tracking sample events and movement in LIMS
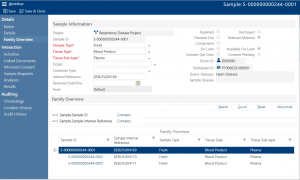
Whether you’re splitting a sample to create aliquots or creating derivatives the LIMS automatically creates an audit history of each sample activity. Plus, when creating aliquots and derivatives the system also maintains the sample genealogy – with no limit on levels. Presenting the sample genealogy in an easily navigable tree.
What’s more, when you create aliquots and derivatives the LIMS copies the parent’s movement history to the children. So, you have a complete picture of what happened with the parent before the aliquot was taken as well as what happened after to the individual aliquot.
But the LIMS doesn’t just audit sample information. Achiever Medical LIMS also helps you effectively manage changes to the patient’s status including consent withdrawals. Achiever Medical LIMS captures the withdrawal date and automatically presents you with all the samples donated. Irrespective of where you’re currently storing or using them. This also extends to include all derivatives and aliquots even if they’re part of a pooled or composite sample. This enables you to locate the samples easily and manage them in line with the patient’s wishes. These samples are updated and completely traceable within the LIMS while making sure that users can’t select them for use.
Working with collaborators including outsourcing laboratory sample activities
Many successful research studies, testing projects and clinical trials involve working closely with other teams and/or organisations. Whether these are internal or external collaborations, they may involve you transferring your samples between multiple locations. Keeping track of the samples you’ve received and sent, where and when, can be especially difficult if you’re using a spreadsheet.
Achiever Medical LIMS’s sample receipt and dispatches functionality helps you keep a close eye on which samples you’ve shipped to other departments or organisations. As you dispatch samples you can record details about the courier, delivery address and contact as well as the expected delivery date. You can also record when your samples arrive at the destination. Plus, if you do encounter any issues you can capture these and detail how you resolved the problem.
Similarly, you can record samples that you have received. What’s more, if you send your samples to another lab for processing there’s an audit trail for shipment and returns.
Auditing and tracking sample movements in LIMS to support regulatory compliance
Traceability of samples used in research, testing and clinical trials is essential for the reproducibility of any outcomes as well as meeting any regulatory compliance.
Achiever Medical LIMS’ automatically auditis all sample events for a complete event history against every sample. Plus, you can also see who carried out the activity. As a result, you have a complete chain of custody and you can retrace every step of each sample’s journey.
Likewise, Achiever Medical LIMS allows you to track a sample’s current and previous locations. Even if you’ve shipped your sample to another location for processing or storage.

Comments are closed.