In a clinical research lab or Biobank, it’s vital you know exactly what biological samples you’re working with and where they came from. Understanding a tissue sample’s provenance helps you assess how you can best use it. And, critically, if it’s safe to use. If this information includes patient consent, then you also know how you can actually use it. If all you know about your samples are their sample types, containers and volumes then that doesn’t really tell you very much. Achiever Medical’s core LIMS functionality helps you track and analyse each sample’s genealogy. Giving you the full picture about every one of your samples, so you can make the most of this very valuable resource and ‘Make Every Sample Matter’.
Tracking the complete genealogy of sample aliquots and derivatives in LIMS
Tracking sample aliquots and derivatives can be difficult when you’re using spreadsheets or in-house built systems. Sometimes aliquots are assigned the same reference as the original samples – making it tricky to work out which sample is which.
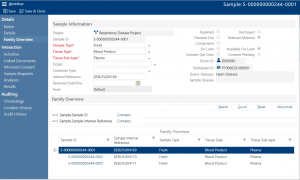
Using Achiever Medical LIMS’ ‘out-of-the-box’ functionality you can split biological samples to create aliquots or derivatives. Each newly created sample is automatically linked to its original sample. In addition, the parent sample has a “split” audit record created logging the date/time and user. From every sample you can view a complete sample family tree with details of sample aliquots, parents, grandparents, great-grandparents and so on. What’s more, the history from the original sample is recorded against each newly created aliquot or derivative. So, you have a complete audit trail on each aliquot.
In addition, the system automatically assigns each new sample a reference number containing part of its original sample’s reference. So, you can see at a glance whether you’re looking at the original sample or an aliquot.
Plus, if you’re working on a study where you’re regularly creating the same aliquots or derivatives you can define templates. These outline the number and types of samples you want to create from each original sample. You can also choose whether the original sample is being depleted as part of the process. All this not only helps ensure consistency and maximum data quality but also saves you valuable time.
Preparing to receive samples aliquots and derivatives
If you’re expecting to receive samples that include original samples as well as aliquots and derivatives you can use Achiever Medical’s bulk import tool to import the data from CSV or Excel files. As part of the import the system automatically creates the corresponding family links and audit trail.
In addition, if you need to capture details of samples that you’re expecting to receive in advance of their arrival into your facility you can do this with Achiever Medical’s pre-sample functionality. You can then use the system to pre-print barcode labels for the incoming samples. Once you physically receive the samples you can update the system and check to ensure that the samples you were expecting actually arrived.
Pooling samples
In addition to creating aliquots and derivatives you can also pool samples of the same type from the same donor. You simply select the samples to include and Achiever Medical will create the relevant links and automatically create an audit trail to indicate what has happened with each of the affected samples.
Creating and working with Tissue Microarrays (TMA)
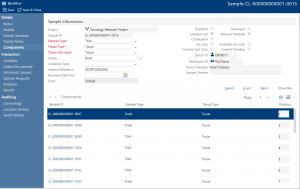
If you work with Tissue Microarrays you can easily set these up in Achiever Medical. Simply choose the samples that make up the TMA and the system will automatically update and link them. You then just enter each sample’s position in the TMA. Plus, every time you take a slice from the TMA the software automatically creates a linked “child” sample for the new segment of each core. So, you maintain the complete genealogy for each core as well as for the TMA as a whole.
What’s more, if one of the donors withdraws consent, Achiever Medical automatically shows you where their samples are including if they’re part of a TMA. So, you can then remove the relevant core and process as part of your Standard Operating Procedures (SOPs). All this helps you meet regulatory compliance.
And it’s not just sample management – it includes donors too
As well as maintaining links between samples Achiever Medical also provides extensive donor profiling capability. This includes donor history and information around diagnosis, treatments and diseases. Plus, the laboratory information management system also provides patient informed consent management. Helping you to meet regulatory compliance, such as the Human Tissue Act (HTA), more easily.
Armed with this information you can be more specific about samples you are providing and using in research. Meaning research is more targeted.
A final thought about tracking sample genealogy using a LIMS
Understanding your samples, their origin and what’s happened to them helps you assess their viability for use in research. This can have a direct impact on research as well as your confidence in any results.
By tracking sample genealogy in LIMS, you can make sure you’re using the most appropriate and best samples for research. Helping to ‘Make Every Sample Matter’.

Comments are closed.