Sample testing capacity within the UK has increased significantly, and unbelievably rapidly, since the start of the COVID-19 pandemic. Time and accuracy are of the essence when it comes to tracking and tracing COVID-19 samples, donors, and potential contacts. With growing volumes of samples and an ever-changing landscape as countries start considering strategies on living with COVID-19 and its variants, the need for managing and processing COVID-19 samples will remain for some time. Is it time to consider a longer-term solution like a LIMS for managing your COVID-19 samples and testing process? Or even for tracking any surplus samples with appropriate consent that you or others could use to provide insight for long COVID, treatments for possible future variants or even other diseases?
Achiever Medical is a web-based, process-driven laboratory information management (LIMS) currently used by UK trusts and academic research institutions to support COVID-19 sample testing as well as manage the ongoing tracking and management of samples and donors.
1. Receiving COVID-19 samples
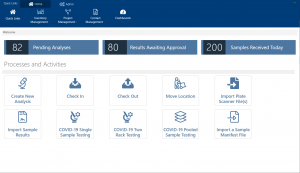
Whether you receive sample and donor data via a CSV file, spreadsheet or you physically receive samples in your lab, Achiever Medical LIMS helps you record your sample, donor, and consent information using your standard terminology and classifications. You can register sample data manually through a simple data entry screen or in bulk via the data import tool. Either way the LIMS prompts you to select from predefined options, notifies you of any missing mandatory information and standardises number and date formats.
The LIMS helps improve your data quality, integrity and structure by making sure you enter data consistently. As a result, it’s easier for you to find and analyse your samples and donors in the future.
2. Processing and analysing samples
Once you have successfully received samples you may want to store them until you have the appropriate number to analyse. Using Achiever Medical LIMS, you can define, manage, and monitor multiple storage units with diverse configurations across multiple sites. The LIMS displays the storage set up, its contents and any remaining capacity in a visual format as well as in a grid.
Whenever you’re ready to start analysing your samples, you may want to aliquot the samples first to create the required sample volume for the test. You can check the newly created aliquots into the appropriate plates and assign to a test run. As you carry out processes in the LIMS using its purpose-built workflows, it automatically audits every event for complete traceability. What’s more, when aliquoting samples, the LIMS automatically creates a link to the sample’s parent for a complete sample genealogy.
For increased testing capacity you can create sample pools made up of multiple aliquots. Each sample within the pool is linked to its original sample and donor for ease of identification. Plus, you can add positive and negative controls to the plate to monitor quality and instrument calibration.
3. Managing COVID-19 sample test results in LIMS
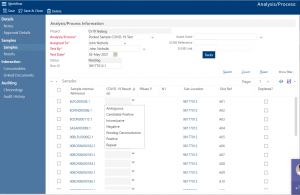
You can record the results and analyses against each sample or sample pool. As you enter results against the pool, the LIMS highlights potential positive candidates for retest. The LIMS also integrates with plate-scanners and instruments to automatically import results data as a result reducing manual data entry and data duplication.
The LIMS can automatically calculate the overall sample status, such as Positive, Negative, Inconclusive, using predefined parameters. Authorised lab technicians can manually adjust this status as required. Achiever Medical LIMS automatically audits this data, so any adjustments are visible. An authorised lab manager or technician can then approve the final status.
Any samples with an overall status of Inconclusive can be retested as required. Likewise, the original samples relating to any positive candidates within a pool sample can be aliquoted and retested with all results visible against each sample within the sample family.
4. Communicating results
You can use Achiever Medical LIMS’ communication activities to automatically send email or SMS notifications to those who test negative.
Alternatively, the LIMS can automatically create telephone call activities for your contact team to get in touch with those testing positive to provide support, advice, and guidance on what to do next.
Achiever Medical LIMS records all communication history against the donor (person) for complete visibility and transparency.
5. Monitoring variants, trends, and throughput
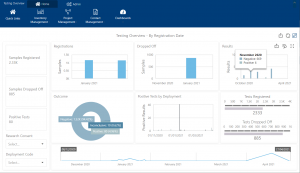
Interactive dashboards present results visually to help you identify trends and progress. You can easily drill into the details behind the figures to gain a more in-depth understanding. What’s more you can simply slice and dice the data to analyse specific data sets. This could include analysing samples from females under 30 or residents in a certain location.
All dashboards can be output in different formats including PDF and Excel for further review and analysis.
6. Beyond COVID-19
You may have surplus samples once you’ve finished testing for COVID-19 that you’re storing. Depending on the consent in place you may be able to use those samples for other approved research purposes. This might include your own research studies or offering them to approved collaborators. Achiever Medical LIMS enables you to track and manage these COVID-19 samples which also includes providing an online Researcher Portal where collaborators can source and request samples. Plus, you can link to the UKCRC online Tissue Directory to further publicise your samples.
What’s more, using Achiever Medical LIMS you can easily add new studies, sample definitions, storage units and consent options. As a result, other labs across your institution can manage, process, audit and analyse non-COVID-19 related samples. Centralising, standardising, and providing complete traceability for all samples in your organisation to improve efficiency, quality and compliance.

Comments are closed.