Consumables are essential to the operation of modern laboratories. Consumable quality or absence can cause risk, delays and inefficiencies that can impact outcomes as well as reputation. This is especially significant for those carrying out time-critical processes in forensic, testing and research labs. Using a laboratory information management system (LIMS) to manage and track your consumables reduces the likelihood of absent and invalid consumables. Helping improve your lab’s efficiency and productivity.
Improve efficiency for time-sensitive laboratory tests and research
Rapid turnaround times are vital in testing and forensic labs. Any delays can result in potential risks to public health, impact individuals’ lives and safety as well as lead to increased costs and consequences for stakeholders, customers, and suppliers. Likewise, delays to personalised medicine research could negatively affect patient care and outcomes.
Identifying missing or expired consumables at the point when you need them is too late, resulting in the postponement of work until you can reorder them. What’s more, you may need to validate consumables before you can use them – which means more time lost.
By using a LIMS system to manage your consumables including stock levels you can make sure you have the quantity available to complete your lab operations.
Achiever Medical LIMS, for example, enables you to set minimum stock levels and decrements the quantity of stock available whenever you use or plan to use the consumable. The LIMS automatically sends email alerts to the relevant personnel as minimum stock levels are reached. An interactive consumables dashboard also tracks stock levels, future requirements, expiration dates, and usage.
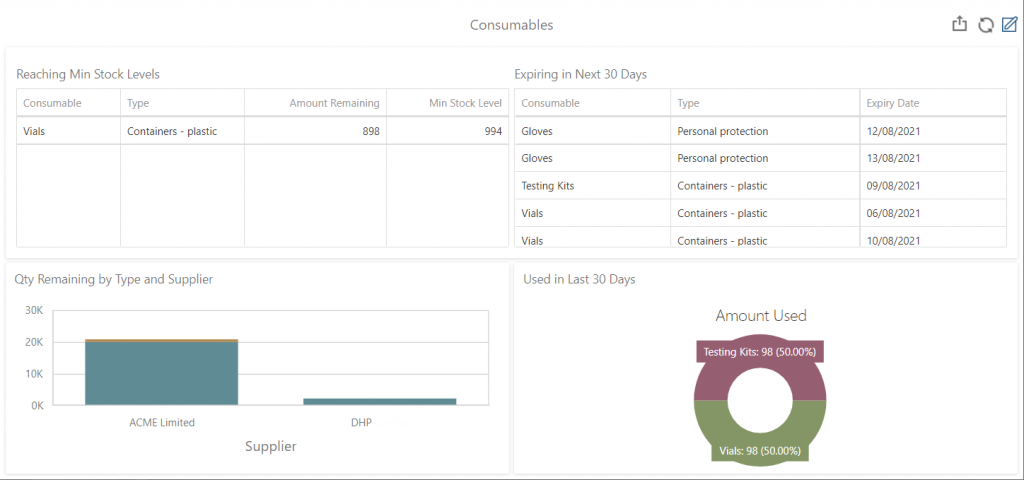
Achiever Medical LIMS Consumable Management Dashboard
Improve quality control with LIMS consumable management
The quality of the equipment and consumables you use in an experiment or analysis could invalidate any outcomes and results. A LIMS can help you track consumable lot and batch expiration dates. As a result, the system can prevent you from adding expired consumables against an experiment or analysis and even send you notifications when the consumable is due to expire. Plus, you can link the supplier against each consumable batch for quality control as well as contract and relationship management.
What’s more, a modern LIMS like Achiever Medical enables you to validate consumable batches in the system. You can track validation outcomes as well as any reasons for failure. Also, you can choose to raise a corrective and preventative action (CAPA) in the laboratory information management system to capture actions taken to resolve any issues. You can then monitor the results through the LIMS’ data visualisation tools to help identify patterns and trends.
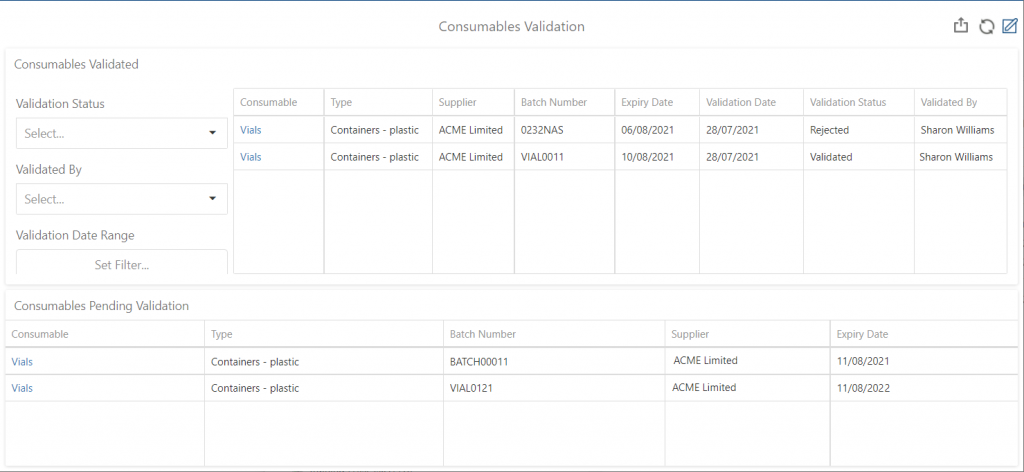
Achiever Medical LIMS Consumable Validation Management
Optimise resources to improve productivity
Your resources are valuable, and you want to make sure you’re using them optimally. Consumables that have expired cost you double – once as a result of not using them and again for storing them. What’s more, you may not know where all your consumables are stored and under what conditions and protocols.
You can lose valuable time searching for consumables or waiting to receive items on order. The delay may also result in you having to store samples until you can process them – which is a further cost.
A LIMS can help you identify which consumables you already have available, where they’re stored, and which are due to expire. Also, the system can provide information on planned work and any low stock levels, so you can order items in advance of when you need them.
Similarly, you can identify consumables you use less frequently to minimise stock numbers to only those that you’ll use well within the stated expiry dates.
Improve sample management and processing
You could hold consumable data in a stock control system that is separate from your sample information. Although you’ll be missing out on the benefits that holding consumable details alongside sample and lab processing data in a LIMS gives you such as complete traceability of sample and consumable usage.
In a LIMS you’re able to see when, why and how you’re using the consumables as well as identify what consumables you’ll need for future scheduled activities. What’s more, you’re able to see exactly what you’ve used to process each sample. Giving you greater transparency, reproducibility and confidence in your findings and results.
Increase compliance with ISO 15189 and ISO 17025
Managing consumables and complete traceability of sample events is required to meet quality accreditations such as ISO 15189 and ISO 17025.
ISO 15189 requires a documented consumable inventory management, storage, and usage procedure as well as the ability to track incidents.
ISO 17025 requires evidence that a laboratory can provide consistently valid results. This means complete traceability of all sample events including consumables, instruments and reagents used.
Both standards also want evidence that only appropriately trained personnel are carrying out highly skilled activities.
A LIMS provides evidence to meet these accreditations as well as other compliance and regulatory requirements. It does this by managing and tracking data on samples, consumables, instruments, processes, and personnel.
Using a LIMS to support consumable management
All labs are striving for repeatable, consistently valid results. For complete sample traceability you need to know exactly how you collected, stored, processed, and destroyed the sample. This also includes the who, how, when and what. Without these vital details it can be challenging to replicate the exact test or experiment conditions.
A LIMS provides a 360° view of each sample – its journey, lifecycle, and genealogy. By tracking consumables, equipment, and processes in a LIMS you can evidence what has happened to a sample. This includes the what, why, when, who and how. Giving you evidence for compliance – but more importantly confidence in your outcomes. For further detailed data you can also consider linking a LIMS to your Electronic Lab Notebook (ELN).

Comments are closed.